VBI Vaccines is initiating a voluntary nationwide recall of all remaining PreHevbrio® [Hepatitis B Vaccine (Recombinant); NDC 75052-001-10] (“PreHevbrio”) due to the bankruptcy of the company and termination of operations.
Further distribution or use of any remaining PreHevbrio vaccine by healthcare providers or others must cease immediately.
VBI Vaccines Inc. and its affiliates commenced a proceeding under Canada’s Companies’ Creditors Arrangement Act, R.S.C. 1985, c. C-36, as amended (the “CCAA Proceeding”) on July 29, 2024, in the Superior Court of Justice (Commercial List) in Ontario, Canada. VBI Vaccines (Delaware) Inc. and certain other affiliates filed petitions and a motion under chapter 15 of the Bankruptcy Code for recognition of the Canadian Proceeding as a foreign main proceeding on July 30, 2024, in the United States Bankruptcy Court for the District of Delaware (together with the CCAA Proceeding, the “Restructuring Proceedings”). In connection with the Restructuring Proceedings, on October 25, 2024, the company issued an initial communication to healthcare providers and the FDA regarding its intent to voluntarily withdraw PreHevbrio from the U.S. market. The company is rapidly winding down all U.S. operations and has permanently ceased distribution of PreHevbrio, a vaccine indicated for the prevention of infection caused by hepatitis B virus in adults.
PreHevbrio was distributed nationwide to distributors and healthcare providers.
COMMUNICATION AND REQUEST FOR DESTRUCTION OF INVENTORY:
VBI Vaccines is notifying its distributors, clients, and healthcare providers by direct mail and is requesting that they further notify or re-notify any potentially affected customers, consumers, and/or patients of this recall. VBI Vaccines is requesting immediate destruction of all existing vials of PreHevbrio in accordance with applicable law, and notification of product disposition to prehevbrio@vbivaccines.com.
The U.S. Food and Drug Administration is aware that VBI Vaccines has permanently discontinued distribution of PreHevbrio.
ADVERSE EVENT REPORTING:
Adverse reactions or possible side effects experienced in connection with the use of PreHevbrio may be reported to Vaccine Adverse Event Reporting System (“VAERS”) either online, by fax, or by regular mail.
- Complete and submit the report Online: https://vaers.hhs.gov/
- Regular Mail or Fax: Download PDF form (https://vaers.hhs.gov/) and fax to 1-877-721-0366, or mail the completed form to:
VAERS
P.O. Box 1100
Rockville, MD, 20849-1100
If you require assistance with reporting, you can contact VAERS by email at info@vaers.org or by phone at 1-800-822-7967.
INTERCHANGEABILITY AND SERIES COMPLETION:
For healthcare providers needing to complete a Hepatitis B vaccination series when the original vaccine administered is no longer available, please refer to the Centers for Disease Control and Prevention (CDC) Hepatitis B vaccination guidelines at https://www.cdc.gov/hepatitis-b/hcp/vaccine-administration/.
HOW TO IDENTIFY PREHEVBRIO:
| Name | NDC Numbers | Label & Additional Information |
| PreHevbrio | NDC 75052-001-01, 75052-001-10 | https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=36e9a878-d6c2-4808-9915-294f47b1fe3d |
PreHevbrio Lots & Expiration Dates:
| Lot Code | Expiry Date |
| B2201V1 | 3/31/2025 |
| B2211V1 | 4/30/2025 |
| B2321V1A | 2/28/2026 |
| B2281V1 | 12/31/2025 |
| B2461V1A | 12/31/2026 |
| B2451V1B | 11/30/2026 |
| B2251V1 | 9/30/2025 |
Carton and Vial Photo:
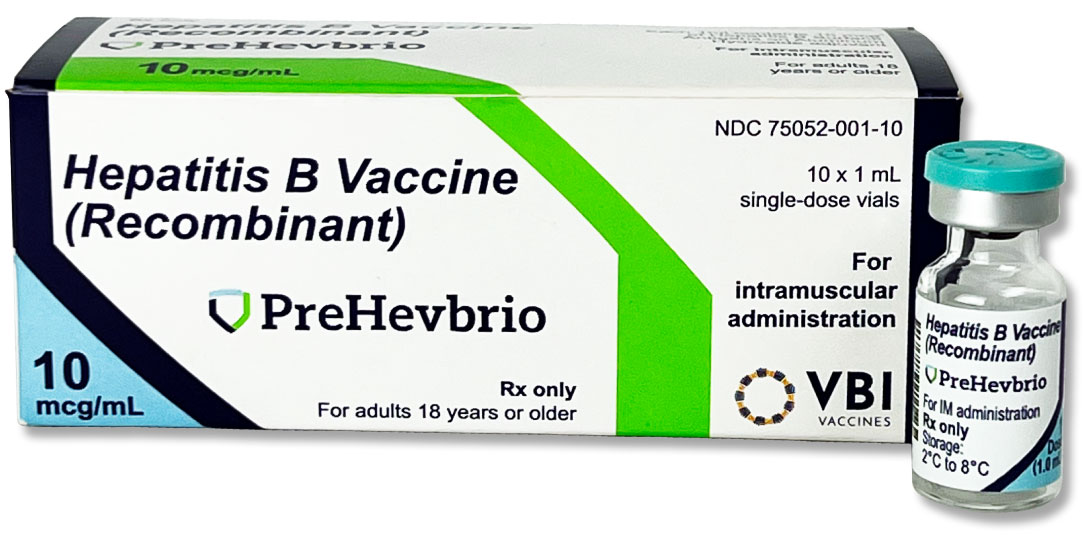
HOW TO IDENTIFY PREHEVBRIO:
- Name: PreHevbrio
- NDC Numbers: NDC 75052-001-01, 75052-001-10
- Label & Additional Information: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=36e9a878-d6c2-4808-9915-294f47b1fe3d
PreHevbrio Lots & Expiration Dates:
| Lot Code | Expiry Date |
| B2201V1 | 3/31/2025 |
| B2211V1 | 4/30/2025 |
| B2321V1A | 2/28/2026 |
| B2281V1 | 12/31/2025 |
| B2461V1A | 12/31/2026 |
| B2451V1B | 11/30/2026 |
| B2251V1 | 9/30/2025 |
Carton and Vial Photo:

ADDITIONAL QUESTIONS:
Healthcare providers and distributors with questions regarding this recall can contact VBI Vaccines at prehevbrio@vbivaccines.com. Consumers who received PreHevbrio should reach out to their physicians or other qualified healthcare providers with any questions or concerns related to the use of PreHevbrio.
Indication & Important Safety Info
INDICATION
PreHevbrio is indicated for prevention of infection caused by all known subtypes of hepatitis B virus. PreHevbrio is approved for use in adults 18 years of age and older.
IMPORTANT SAFETY INFORMATION
Do not administer PreHevbrio to individuals with a history of severe allergic reaction (e.g. anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of PreHevbrio.
INDICATION
PreHevbrio is indicated for prevention of infection caused by all known subtypes of hepatitis B virus. PreHevbrio is approved for use in adults 18 years of age and older.
IMPORTANT SAFETY INFORMATION
Do not administer PreHevbrio to individuals with a history of severe allergic reaction (e.g. anaphylaxis) after a previous dose of any hepatitis B vaccine or to any component of PreHevbrio.
Appropriate medical treatment and supervision must be available to manage possible anaphylactic reactions following administration of PreHevbrio.
Immunocompromised persons, including those on immunosuppressant therapy, may have a diminished immune response to PreHevbrio.
PreHevbrio may not prevent hepatitis B infection, which has a long incubation period, in individuals who have an unrecognized hepatitis B infection at the time of vaccine administration.
The most common side effects (> 10%) in adults age 18-44, adults age 45-64, and adults age 65+ were pain and tenderness at the injection site, myalgia, fatigue, and headache.
There is a pregnancy exposure registry that monitors pregnancy outcomes in women who received PreHevbrio during pregnancy. Women who receive PreHevbrio during pregnancy are encouraged to contact medinfo@vbivaccines.com or call 1-888-421-8808 (toll-free).
To report SUSPECTED ADVERSE REACTIONS, contact VBI Vaccines at 1-888-421-8808 (toll-free) or VAERS at 1-800-822-7967 or www.vaers.hhs.gov
Please see Full Prescribing Information.